- Set laser to central wavelength, 970 nm
- Manually adjust pockels to about so beam is just bright enough on card to see
- Check beam is still passing through irises of PPC in bypass mode
- Adjust angle of prism such that beam passes through center of exit iris
- Direct beam into bottom of table periscope, through an iris on the table and the iris on the bottom of the periscope
- Adjust the angle of the mirror at the bottom of the periscope such that the beam comes out of the tube straight. (Could align bottom mirror with test laser to ensure it is in the middle of its travel range). Want to make sure the mirror is not so far off that it the beam is hitting the edge of the tube and you are looking at a dim reflection.
- Do rough alignment of all the gantry periscope mirrors so that the beam remains centered on all of them until the beam hits the first mirror of the entrance periscope.
- Add the quarter waveplates before the gantry periscope on the table, and add the entrance quarter waveplate on the vertical breadboard. Set it 45° to the fast axis to make the polarization circular going into the gantry periscope.
- Mount camera alignment system onto remote focus objective holder. Align beam to pass through two irises on remote focus alignment jig using two translation stages on beam periscope.
- Add the quarter waveplate before the gantry periscope on the table, Set it 45° to the fast axis to make the polarization circular going into the gantry periscope.
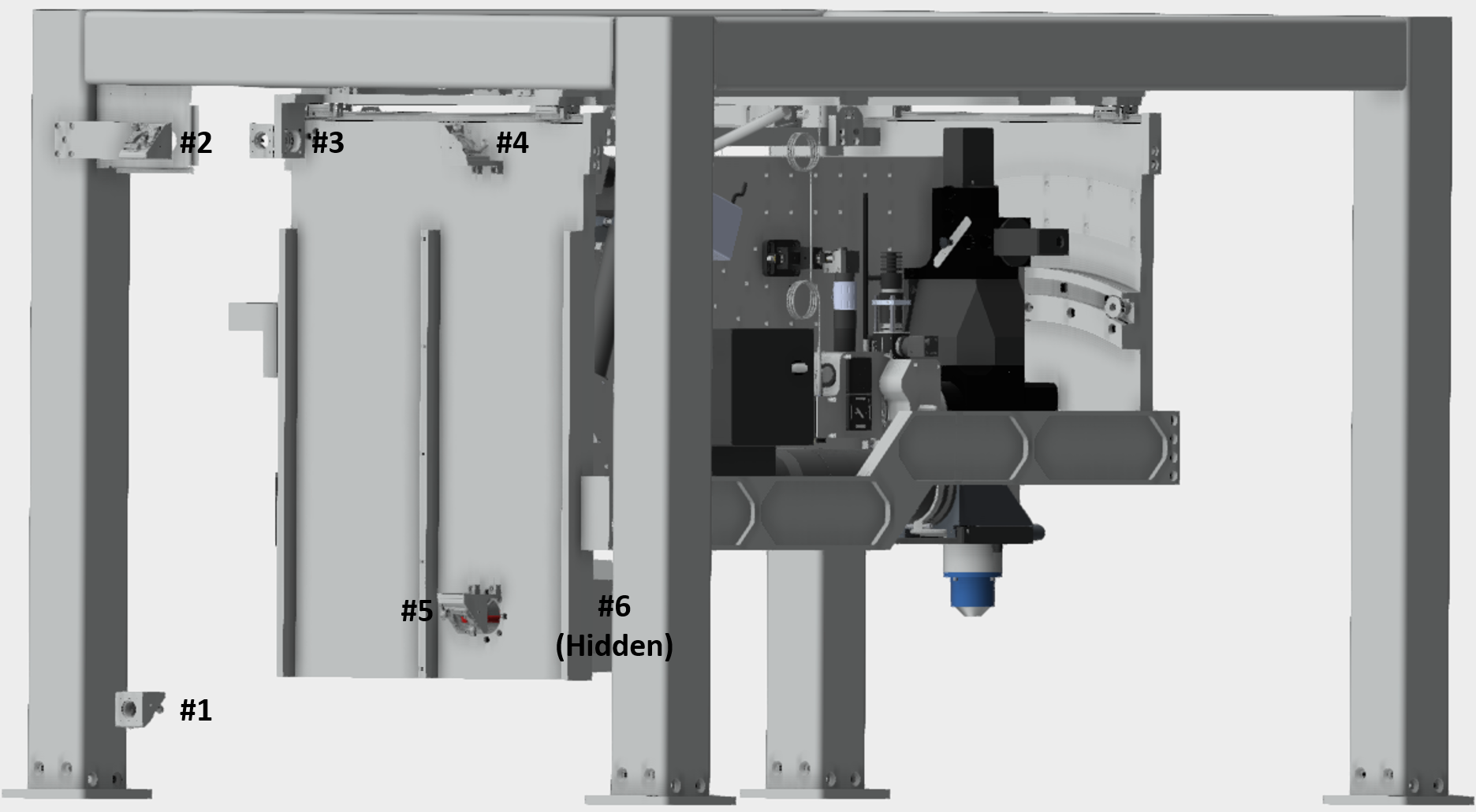
The gantry system translates the microscope along 3 axes (X,Y,Z) and rotation (θ). Ideally, the beam should not deviate during movement of any of the four translations. Any movement results in a deviation of the beam onto the back aperture objective and consequently affects the scanned image. Therefore, a multi-mirror periscope which consists of 6 mirrors (See picture below) was engineered in order to allow sufficient control of the beam alignment onto the vertical breadboard. Completion of this alignment defines the beam propagation on the vertical breadboard to which all vertical breadboard optics should be aligned to.
Coarse alignment of gantry periscope: The first step is a coarse alignment, this just ensures that the beam is roughly going through the center of each of the six periscope mirrors. This safeguards that the beam won’t clip on the housing when fine adjustment commences.
-Adjust the last two mirror from the prism compressor such that the beam is parallel to the plane of the table and hits periscope mirror #1 at 45° directing the beam up to periscope mirror #2.
-Adjust the knobs on periscope mirror #1 such that the beam is roughly centered on periscope mirror #2
-Adjust the knobs on periscope mirror #2 such that the beam is roughly centered on periscope mirror #3
-Adjust the knobs on periscope mirror #3 such that the beam is roughly centered on periscope mirror #4
-Adjust the knobs on periscope mirror #4 such that the beam is roughly centered on periscope mirror #5
-Adjust the knobs on periscope mirror #5 such that the beam is roughly centered on periscope mirror #6
It is essential that the beam does not deviate when any of the four translations of the gantry are moved. In order to confirm this property each axis must be tested and aligned individually. While one could monitor the deviation coarsely by eye for fine alignment a digital readout is essential. Therefore, it is best to use a camera, beam profiler or position sensitive detector to monitor beam deviations.
Place the sensor far away from the periscope. Ideally, this sensor is placed after the remote focus and objective jig. **At this point the telescope, remote focus objective and voice coil should be removed. Use the vertical breadboard periscope to roughly center the beam through both irises from the jig onto the center of the detector. This large separation ensures any small beam deviation will result in a detectable change in the position on the sensor. First, start the gantry to one extreme of its travel and note the location of the beam on the sensor. Next, move the gantry to the other extreme and record its position on the sensor. If the input beam is perfectly aligned to the axis of travel the two positions should not change. In practice some difference will always be observed albeit small since the alignment is limited by the linearity of travel along the axis.
A particular axis alignment is controlled by a specific periscope mirror for linear axes X, Y and Z and 2 periscope mirrors for rotation. These relationships were defined by how the mirror mounts are attached to the gantry. Consequently, there is a particular order and a specific beam alignment to how the periscope is aligned to the mounted breadboard. The order is as followed;
-Periscope mirror #2 for Y axis.
-Periscope mirror #3 for X axis.
-Periscope mirror #4 and #5 for rotation.
-Periscope mirror #6 for Z axis.
- Move gantry system back and forth in y and adjust . Adjust top mirror on periscope gantry such that the beam remains stationary on the camera.
- Move gantry system back and forth in x and adjust . Adjust first mirror inside box (high up) such that the beam remains stationary on the camera.
- For rotation need to control four degrees of freedom, so have to move camera between two locations on the vertical breadboard (near) and (far), second one is , where it is now). The second location is ideally where quarter waveplate goes (near camera). Have to keep beam centered on both. First keep centered on near camera using far mirror, the one that comes next after one used for x, (high up and closer to vertical breadboard). Then keep centered on far one using mirror at bottom of gantry to keep centered on far one. Watch beam move on camera during move, use mirrors to adjust beam position to be at the center of the curvature of the circle traced out by the beam. THIS CAN BE QUITE TIME CONSUMING - IN PART BECAUSE THE GANTRY ANGLE HAS TO BE MOVES BACK AND FORTH.
- Aligned z axis using mirror inside the rotation bearing, keeping beam constant on the camera in the far position.
- All alignments for each axis is iterative. Therefore, it might take multiple iterations before acceptable alignment is achieved. Successful alignment requires patience.
- Add in the other quarter waveplate, set to minimum reflection (maximum transmission) through polarzing beam splitter. Put the card to look at the reflected beam and make it as dim as possible.
- At this point, if the the first quarter waveplate (QWP #1), outside of the microscope cage, is not rotated ideally, then there will be some change in transmitted intensity through the PBS as the microscope rotation is changed. To fine tune the angle of QWP #1, use a power meter to check the beam power after the PBS (remote focus objective is removed) while rotating the microscope back and forth. Observe the power difference, and make small adjustments to the rotation of QWP #1 (rotating QWP #2 each time afterwards to maximize transmission) until the power difference upon microscope rotation is minimized.
- Add in the beam expander. There is an iris on the front, so position . Position so that beam goes through it and with the whole assembly centered.
- Add the beam alignment jig (two 60mm cage plates with irises and long cage rods) to the remote focus objective location, using the RFAlignmentJigInterface (part J003123).
- Move the entire beam periscope assembly such that the beam is centered on two periscope mirrors. Align periscope output beam through the two irises on the remote focus alignment jig and into the camera using the two translation stagesusing the two translation stages on each periscope mirror (which should produce pure translation), and the two tilt knobs on the Polaris mount in the periscope. The beam should be centered on the first iris, and the angle of the beam should coincide with the jig axis (shadow of first iris in beam overlaps with second iris). It helps to use a beam camera to view the centration of the beam on the first iris in the alignment jig (set using the two mirror translations).
- Mounting the voicecoil. Remove the remote focus object so that you don't accidentally damage it. (The remote focus objective and RFAlignmentJigInterface should be off.) The dowel pins are used to guide the voice coil assembly during mounting, but the exact position of the assembly is set using the nudgers. The lateral position of the beam is adjusted by changing the angle of the voice coil assembly with the two nudgers. Do this while looking at the back reflection on a card near the entrance to the large bearing. The yaw position of the beam is then adjusted by tipping the voice coil assembly. This procedure ensures the mirror is perpendicular to the beam. Look at the beam back near where the quarter waveplate
- . If you ever see a beam on the card then you know you aren't aligned.
- Once the voice coil mirror is aligned remove the voice coil and add remote focus objective back on.
- Remount the voicecoil on the vertical breadboard. First, loosen the z-adjust of the voicecoil (screws which go through stock Thorlabs baseplate), and move voicecoil AWAY from where the remote focus objective will be, so that there is not a crash when the voicecoil is mounted. Add the voicecoil back onto the rig, being sure to touch nudgers to set angle properly.
- Now the proper z-position of the remote focus mirror will be set. Turn voicecoil on (read manual and use Hyperterminal, make sure it is enabled (k1 or k3 command) and voicecoil is stiff) and set control voltage to approximately 4V (this is to bias the usage range such that the remote focus mirror is less likely to crash into the remote focus objective if something unusual occurs–note that the LFA2004 that is being ordered specifically for the 2p-RAM has an analog command range of +/-10 V, mapping to +/-2 mm, not the +/- 2.5V range that is mentioned in the manual). The mirror will be slowly advanced towards the remote focus objective (physically, not using control voltage), until the beam exiting the remote focus objective is roughly collimated. If the new, in situ procedure for aligning the resonant mirror is being followed, and PupilRelay1 and PupilRelay2 are not present, then the beam can be seen directly after it reflects off of the polarizing beamsplitter cube. If PupilRelay1 and PupilRelay2 are present, then the beam can be observed in the space between the two y-axis galvo mirrors, where the beam is traveling downward. In this space, the remote focus mirror is in the approximately correct location when the beam size here is 11mm in diameter. At any time, the quarter wave-plate that is closest to the remote focus objective can be adjusted to maximize the beam power traveling through the rest of the microscope
- If the in situ procedure for aligning the resonant mirror is being followed, then it should occur now. Afterwards, add pupil relays 1 and 2 back, following procedure here.
- At this point, mount the MainAlignmentJigInterfaceAssby to the main imaging objective space. This will send the beam out horizontally and the beam should be visible using an IR viewer. Collimate the beam by adjusting the voicecoil control voltage (in ScanImage, the linear scanner offset voltage will be used) until the beam size stays roughly constant while propagating a few meters.
- Add the alignment jig (two 60mm cage plates with irises and long cage rods) to the MainAlignmentJigInterfaceAssby to observe the beam alignment at the imaging objective space. The 3 galvo mirrors should be engaged, set to the 0 position (use Point function in ScanImage), and should have been previously aligned relative to the galvo mounting block. Use the 4 compensator knobs (the resonant mirror translation, and the translation and tilt in two dimensions of the fold mirror that proceeds the galvo mirror assembly) to align the beam to the alignment jig, similarly to how the beam was aligned to the alignment jig when it was in the remote focusing objective space using the 4 knobs on the small beam periscope.
- At this point the beam should be roughly aligned simultaneously to the remote focus objective and imaging objective. However, to achieve good remote focusing performance, the image of the pupil of the remote focusing objective and pupil of the imaging objective need to be aligned (translation-wise) precisely. This requires adjustment of the 4 compensation knobs while looking at imaging performance in real-time. Use the following procedure:
- Remove the MainAlignmentJigInterfaceAssby and mount the imaging objective.
- Prepare slide with large, deep well of fluorescein dye and proper coverglass thickness. This slide needs to be mounted relative to the imaging objective at the right distance, and orthogonal to the optical axis. There are two ways to do this:
- Using AccessoryHolder_Simple (part J005700A). Attach this to the objective, and attach dye pool slide to it, as shown in AccessoryHolder_Simple_Assby.dwfx.
- Taking advantage of the known beam collimation and alignment entering the imaging objective. See video attached below. The slide is mounted to the optical table and approached by the imaging objective, with field curvature correction turned off in ScanImage. Use a full FOV, striped MROI setup in ScanImage such as roiTile10umPixels.roi. With the beam collimated at the imaging objective, the microscope should be lowered until the focus is 355um below the top of the dye pool. This is the natural focus position, as designed, and all imaging of slides or animals should occur at this coverglass-objective separation (+/- 100um, see below) for ideal imaging. Then, the remote focus (fast Z in SI) should be adjusted until the focus is just below the top of the dye pool (depth/V scaling in SI MDF should be adjusted so that the proper values, in um of depth, can be used in SI). A spot smaller than the FOV should be seen in the image, which has an outline that is the intersection of the curved imaging plane with the top of the dye pool. This spot will move in the imaged FOV as the objective-slide relative angle is changed, and should be centered when the optic axis and slide are orthogonal, as long as the beam is aligned to the optic axis at the center of the scan field (previous alignment step). Adjust the objective-slide angle (one axis is accomplished through microscope rotation, and the other can be adjusted by putting the slide on a goniometer or other tilt stage) until the imaged spot is centered.
View file name Alignmenttomicroscopeusingdyepoolimage-1.m4v height 150 - At Janelia, it was found that the two methods above did not agree very well. That is, when the beam was adjusted to be collimated and angularly aligned at the objective, and when using the AccessoryHolder_Simple, the focus was not found to be 355um below the top of the dye pool (there was a ~200um difference from what was expected here), and the spot showing the intersection of the imaging sphere and dye pool top was not centered in the imaged FOV. It's not known what caused these discrepancies, but some amount of compensator adjustment was carried out using both methods of initial alignment, and it seemed that similar final performance should be achievable in either case. It may be a good idea to check both alignment methods, but as long as the discrepancies between them are less than ~250um in distance, and 1 deg in angle, similar final performance can probably be achieved, and it is probably best to use the alignment method that is most like what will be used when doing daily experiments. I.e. if the animal is attached to the optical table, the latter method can be used to align the objective and animal, and should be used to do the compensator adjustment; if the animal is attached to the objective somehow with a position and accuracy similar to that designed into AccessoryHolder_Simple, then the former method should be used.
- A THIRD method for aligning the slide angle is using the Coverglass Alignment module in the accessory optical system. See attached video below. This system can be calibrated using the AccessoryHolder_Simple part, most easily by mounting a piece of glass such as an OD 4 ND filter on it (this will show a reflection that is imaged onto the coverglass alignment camera only from the top surface). With this alignment step, using this system should give an alignment equivalent to mounting the slide onto the AccessoryHolder_Simple part, and using this system should allow a greater alignment sensitivity than the method of observing the intersection of the image sphere with the coverglass bottom. That being said, this extra sensitivity may not lead to much increase in signal level, as the objective-coverglass alignment was not found to very sensitively affect the signal level.
View file name Coverglassalignmentsystem-1.m4v height 150
- Turn on field curvature correction in SI (see adjustment video below), adjust Fast Z (remote focusing) until focus is a little below the top of the dye pool, such that it's easy to see how well the field curvature correction is working. Adjust the field curvature correction radius of curvature (in MDF) and Fast Z Lag Time (in SI Fast Z dialog box) until field curvature correction works as well as possible.
View file name Fieldcurvaturecorrectionadjustment-1.m4v height 150 - With slide and objective properly aligned, begin MROI imaging with field curvature correction on. Set up ~15 small (500 x 500 um) ROIs around the entire volume FOV. At Janelia we use 5 ROIs–top, bottom, left, right, center–in three planes. As to the precise location of the ROIs, they should be close to the edges of the used FOV, so that performance at these locations is optimized, but not TOO far out. Edges at 2.4mm from the center, and planes at 100um below the top (make sure no ROIs image above the top of the dye pool), 300 um, and 600 um should work fine. See example roi group roigroupOptimize_NotFullResWidth_ToEdges_100-500-1000.roi (these perhaps go too deep–we were checking limits of remote focusing performance). Make sure there are no bubbles, artifacts, or dye pool walls near any of the ROIs, as these may wander into the ROIs during optimization. Image all ROIs in MROI mode, and with a few frames rolling average to reduce noise, then adjust compensator knobs (putting arm through small door in side of cage while room is darkened) to maximize the average signal over all ROIs. The SI user function userFunctionBar.m can be used to display the integrated signal across each ROI in real-time (first n bars, where n is the number of ROIs), plus the average signal over all ROIs (n+1 bar). At first, use the bars function with norm=0 (parameter in userFuntionBar.m file) to simultaneously roughly maximize the signal across all ROIs and make sure that the signal is not highly non-uniform across the ROIs. It will probably be impossible to make the signal highly uniform across the ROIs, and there may be some trade-off between uniformity and maximum total average signal. To fine tune, switch to norm=1 to see the signal relative to the last acquisition made. See video below. Here is the basic compensator adjustment strategy:
- There are two sets of adjusters which works more-or-less orthogonally to each other–the translation and tilt along one axis, and the translation and tilt along the other axis. Look at the fold mirror mount to figure out which tilt goes along with which translation axis. ALL 4 knobs can misalign the remote focus objective and imaging objective pupils, so the signal intensity of the ROIs will depend on each of them sensitively. However, it will be found that for each pair of adjusters there is a global maximum that can be found by tweaking one knob, remaximizing with the other, and then looking at how the signal level changes as this is repeated. In effect, this is adjusting the center of the scan range for this axis while keeping the pupils aligned, and this has a much smaller signal sensitivity than the pupil alignment itself. Iterate back and forth a few times between optimizing with two adjusters, and then the other two.
View file name Compensatoradjustmentsetup-2.m4v height 150
- There are two sets of adjusters which works more-or-less orthogonally to each other–the translation and tilt along one axis, and the translation and tilt along the other axis. Look at the fold mirror mount to figure out which tilt goes along with which translation axis. ALL 4 knobs can misalign the remote focus objective and imaging objective pupils, so the signal intensity of the ROIs will depend on each of them sensitively. However, it will be found that for each pair of adjusters there is a global maximum that can be found by tweaking one knob, remaximizing with the other, and then looking at how the signal level changes as this is repeated. In effect, this is adjusting the center of the scan range for this axis while keeping the pupils aligned, and this has a much smaller signal sensitivity than the pupil alignment itself. Iterate back and forth a few times between optimizing with two adjusters, and then the other two.
- At any point in the MROI signal optimization imaging, the position of the retroreflector in the prism pair compressor can be optimized. This should be done. Due to the properties of the retroreflector, it can be freely moved without adjusting the beam alignment downstream of the PPC. Unless this was already set very close to the optimum location (optimum GDD), a large gain in signal level should be seen.
- After optimizing the signal using the 4 compensator knobs, try adjusting the galvo Y1 gain adjustment, which should in effect adjust the location off the Y galvo scan pupil location. If the signal can be improved with this adjustment, then iterate back to the 4 alignment compensator knobs again.
- Finally, see if the signal can be improved through adjusting the beam alignment entering the microscope using the 4 knobs on the small beam periscope. Ideally, the sensitivity should be low here, and the knobs should be at or near their optimum positions, since moving these should misalign the beam going into the remote focus objective. If a large improvement in signal can be made, then perhaps the beam got misaligned before starting the entire procedure.
- NOTE! After adjusting the compensator knobs, the center of the scan range will probably no longer be aligned as well to the optical axis of the imaging objective. With the field curvature correction off and the focus slightly below the surface of the dye pool, look at where the image sphere/dye pool top intersection spot is located in the FOV. If the location of this spot is used in the future to align the objective-coverglass angle for imaging, then the current position (NOT center position) should be used as the goal.
- The alignment should now be complete, and PSFs can be measured using, for example, beads mounted in agar.
- Over a period of weeks, the beam entering the microscope may be found to wander slightly, probably due to alignment drift coming out of the laser. This should be monitored at the entrance pupil of the beam expander on the raised microscope baseplate, for example by looking at the shadow of the pupil relative to the beam using a NIR beam card where the beam enters the remote focus assembly. The fix this alignment, multi-stage periscope mirror #1 (see image above) should be used.